32+ Titration Calculation Example
Web The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide which react in a 12 moles. The examples will make this clear.

Ppt Chapter 17 Complexation Reactions Amp Titrations Powerpoint Presentation Id 6555548
The first example involves a strong.
. Web To use titration methods to analyze solutions quantitatively. For each trial 250 mL of 0050 molL of a sodium carbonate. Web A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.
A student performs a titration experiment to determine the concentration of a solution of nitric acid. Web The following example exercise demonstrates the computation of pH for a titration solution after additions of several specified titrant volumes. Calculations to determine the concentration of the analyte are generally the next step in the titration process.
In an acid-base titration the titrant. Web Calculation of relative formula mass - Tyler de Witt video. MCAT Unit 9.
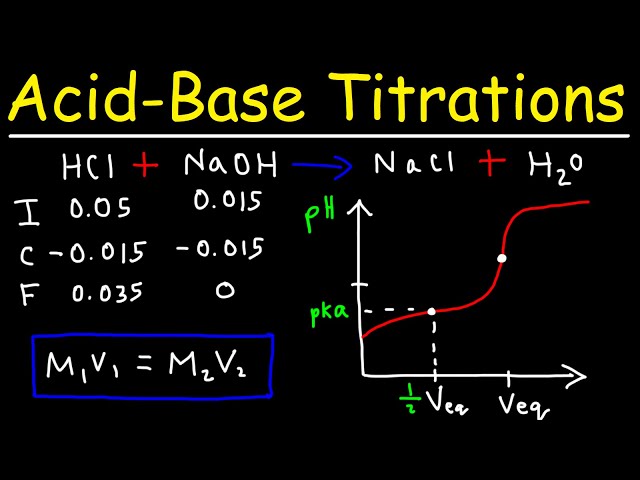
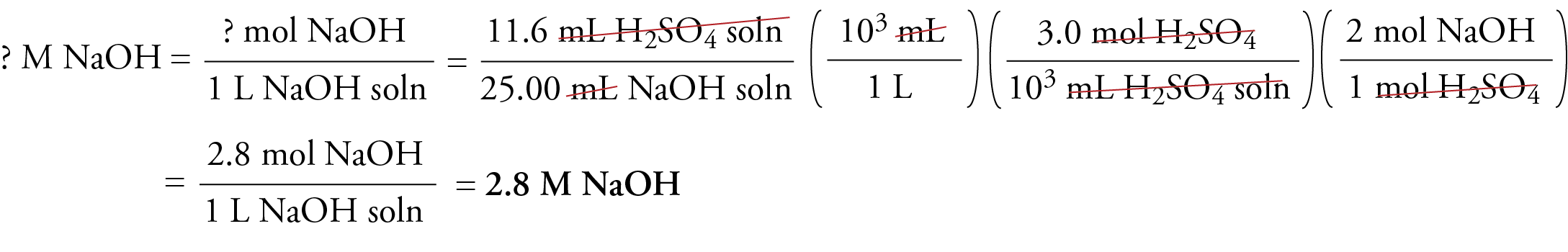
Web Finally apply the final stoichiometric calculation using the moles of solution B and given volume of solution B to calculate the molarity of solution B needed to neutralize solution. N mol C mol L V L and the amount of titrant can be used in the usual. Web In a titration a solution of known concentration the titrant is added to a solution of the substance being studied the analyte.
Web The amount of added titrant is determined from its concentration and volume. Determining solute concentration by acidbase titration. Web It explains how to solve acid base titration problems using a formula and a conversion process in typical solution stoichiometry problems.
This video contains plenty. 233 Triple only describe how. Web Common examples are methyl orange envelope range between 3 and 5 pH or bromothymol blue envelope range between 58 and 76 pH since the color change.
Web For example consider the titration of a 250-mL sample of 0100 M ceHNO3aq the analyte with 00500 M ceNaOHaq the titrant. Web Calculations and Graphing. Water of Crystallisation calculation example.
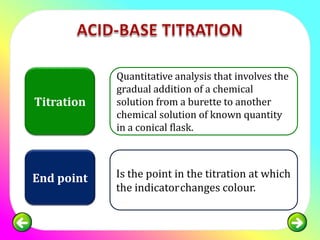
Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Web Titration Calculation Examples Lesson Summary Frequently Asked Questions How do you perform a titration step by step. To determine the amounts or concentrations of substances present in a sample chemists use a combination of.
Example 1 250 cm. Web The results of a titration can be used to calculate the concentration of a solution or the volume of solution needed. If the compound reacts rapidly and completely with.
Calculating a concentration Worked example In a titration. Empirical formula calculation example. At the equivalence point in an acid-base.
After the titrant is in the burette. Web Suppose for example we know the identity of a certain compound in a solution but not its concentration. Web Updated on November 26 2019 An acid-base titration is a neutralization reaction performed in the lab to determine an unknown concentration of acid or base.

Titration Calculation Example Youtube
Titration Problems

Titration Calculation Example Youtube

Chiral Discrimination In The Binding Of Tris Phenanthroline Ruthenium Ii To Calf Thymus Dna An Electrochemical Study Bioconjugate Chemistry

20 Complexometric Titration Pdf Titration Chemistry

Titration Practical And Calculation Naoh And Hcl Youtube
Chapter 7 Acid Bases Part 4 Ppt
:max_bytes(150000):strip_icc()/chemistry-research-172591498-58bc66a15f9b58af5c6c24e1.jpg)
Acids And Bases Titration Example Problem

Titration Calculations Youtube

Acid Base Titration Curves Ph Calculations Youtube
Acid Base Direct Titration Calculations Chemistry Tutorial

Titration Equation Calculations Examples Study Com

Question Video Calculating The Mass Of Solute In A Solution Via Titration Nagwa

Complexation And Precipitation Reactions And Titrations Ppt Download
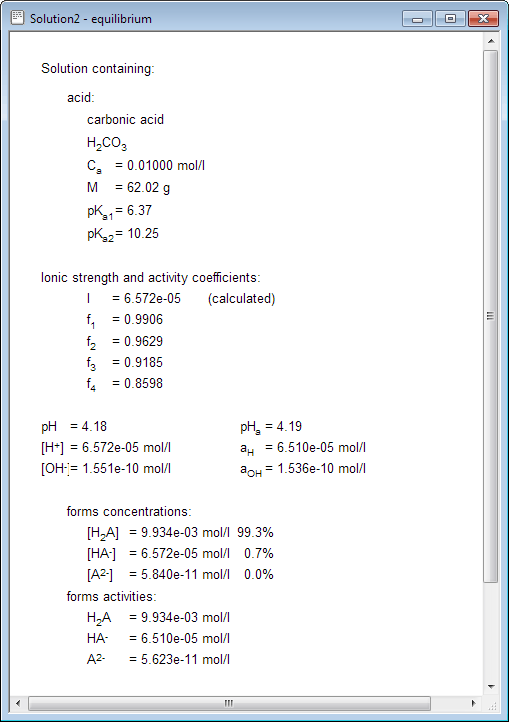
Acid Base Titration And Equilibria

Titration Practical And Calculation Naoh And Hcl Youtube

Chapter 11 Edta Titrations Ppt Download